ABSTRACT
Temozolomide inhibits the proliferation of neoplastic cells in the treatment of astrocytoma / glioblastoma. It may induce significant adverse drug reactions such as leukaemia, aplastic anaemia, agranulocytosis, and allergic responses.
This report presents a case of a 57-year-old male patient admitted to our hospital with persistent headache. Astrocytoma was diagnosis through CT and MRI scans. Right subtotal resection of the tumor was done. Radiotherapy and temozolomide therapy were initiated. The patient was readmitted with strong headache, hyperpigmentation, and acute gastritis. Relief was there when Temozolomide was stopped and a course of Methylprednisolone, Paracetamol, Ondansetron, Levetiracetam, and Pantoprazole was commenced.
1.Introduction
Astrocytomas, specifically glioblastoma (GBMs), represent the most aggressive primary brain tumours with the most unfavourable patient survival prognosis. Gliomas represent a prevalent and highly malignant kind of primary neoplasms affecting the central nervous system in the adults. The classification of gliomas is based upon the specific glial cell from which they originate. Astrocytomas and GBMs pertain to gliomas that arise from astrocytic cells, while oligodendrogliomas originate from oligodendrocytes and ependymomas arise from ependymal cells (Hirtz et al., 2020). The condition is still incurable, with a 15-month median survival time. Radiation therapy (RT) and medical management/chemotherapy are currently the conventional approaches for controlling glioblastoma. These approaches consider maximum surgical resection because glioblastoma infiltrates surrounding tissue and cannot be eliminated (Alifieris & Trafalis, 2015).
Presently, the best course of treatment for newly diagnosed glioblastoma involves a complex initial course of care, i.e., maximally safe surgical resection, concurrent temozolomide chemotherapy (75 mg/m2 oral daily for 6 weeks), and six cycles of adjuvant temozolomide monotherapy (150–200 mg/m2 oral daily for 5 days each 28-day cycle) (Stupp et al., 2005).
Temozolomide is an alkylating agent that is used for the treatment of severe brain tumours. This drug has been utilised for more than two decades in clinical settings, mostly for the management of neurological malignancies such as glioblastoma and astrocytoma (Gilbar et al., 2021). It modifies DNA or RNA at specific sites on guanine and adenine by adding methyl groups. These methylated sites can be fixed, removed, or dealkylated using DNA mismatch repair (MMR), base excision repair (BER), or demethylating enzymes like O6-methylguanine methyltransferase (MGMT).
Glioblastoma cells are sensitive to temozolomide when MMR is active, while resistant to MGMT, APNG, and BER proteins (Lee, 2016). Chemotherapy-induced myelosuppression, a prevalent adverse reaction, due to the cytotoxic effects of drugs, destroys or decelerates the proliferation of highly active cells. While this therapeutic intervention specifically targets malignant cells, it also adversely affects the viability of normal bone marrow cells. However, this recovers within a month. Leukaemia, aplastic anaemia, agranulocytosis, sensation of “pins and needles” in the arms and legs and an allergic response that may result in rash, low blood pressure, asthma, shortness of breath, and swelling of the face or throat are among the major adverse effects of chemotherapy.
This case study is based on temozolomide induced acute gastritis seen in a man diagnosed with glioblastoma. He had surgical resection, followed by concurrent administration of temozolomide and radiotherapy, which led to hyperpigmentation.
2.Case presentation
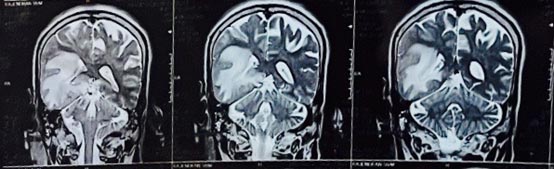
A 57-year-old man consulted his primary care physician in June 2023, due to a persistent and intense headache lasting for 20 days, altered behavioural and mental state. Computed tomography showed a poorly defined ring of haemorrhagic focus in right temporal region and moderate disproportionate perifocal edema causing effacement of ipsilateral right lateral ventricle and mild midline shift to left with thin rim of extra axial haemorrhage and few air foci along right temporal region and right fronto-parietal convexity.
Magnetic resonance imaging demonstrated a mass effect in the form of effacement of overlying cortical sulci, narrowing of right lateral ventricle with midline shift of 7.3 mm towards left side. Right uncal herniation was noted with effacement of right ambient cistern and deformed right cerebral peduncle. A lesion was also seen exerting mass effect on the third ventricle. There were also multiple lesion foci of SWI blooming and foci of restriction diffusion which indicated glioblastoma. He was commenced on methyl prednisolone and levetiracetam.

The ultrasound revealed grade 1 hepatic steatosis(fatty liver). On 19th June 2023, right pterional craniotomy and subtotal resection of tumor was done. A short-course radiation (40 Gy in 15 fractions over 3 weeks) was commenced. Temozolomide (75 mg/m2 for 21 consecutive days) was given from June 29th to July 19th.
The patient began to exhibit hyperpigmentation on the right side of the head, at the surgery site while the was nearing completion.
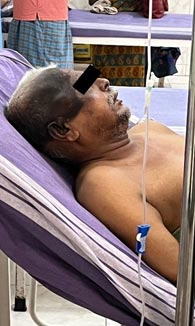
Since he was taking a long time to get better, plans were made to discharge him. On the 18th September, the patient was readmitted to SRM Hospital with frontal / occipital headache, fever and cough for 2 days, giddiness for 3 days and 1 episode of hematemesis. His hemoglobulin level was 9.7g/dl. He was given methyl prednisolone, ondansetron, folic acid, levetiracetam, paracetamol and pantoprazole. Over the next 3 days, body temperature improved. But one episode of hematemesis was there. He was transferred to Medical Gastroenterology on 21st September. UGI scopy gave the impression of acute gastritis (Picture 4).
Same medicines were continued. Haematology consultation on 22nd September suggested acute gastritis likely induced by temozolomide. All the tests for dengue, HIV, hepatitis B, and hepatitis C were normal. The test for anti-nuclear antibodies was negative. The CT scan showed nothing that could explain how he looked. They recommended to stop the temozolomide and after a day, hematemesis gradually reduced and stopped. Hence, temozolomide treatment was not given as a follow-up.

3.Discussion
Acute hemorrhagic gastritis (AHG) is a significant cause of upper gastrointestinal (UGI) bleeding, including roughly 25% of cases seen in endoscopic investigations. Most individuals with AHG exhibit preexisting risk factors, including but not limited to alcohol consumption, portal hypertension, the utilization of nonsteroidal anti-inflammatory drugs or chemotherapy over a short or extended period, as well as physiological stress experienced during hospitalization in an intensive care unit due to serious life-threatening illness or trauma (Lee, 2016).
Patients with AHG, characterised by a serum haemoglobin level less than 8.0 g% and an average transfusion demand of 21 units of blood, have a mortality rate as high as 50%. Patients often exhibit symptoms indicative of a decreased haemoglobin level, as well as hematemesis (Naranjo et al,1981). The stomach mucosa is often affected by medication-induced damage, with the antrum being the most common site of occurrence. However, it is also possible to see patchy or widespread gastropathy in certain cases (Panarelli 2014).
Potential symptoms that individuals may experience include weariness, pallor, weakness, fever, chills, and pharyngitis. Various pharmacological preparations have been used in the treatment of brain tumours, including anticonvulsants such as carbamazepine and phenytoin, as well as anti-infectives like sulphonamides.
The timely identification of drug-induced acute gastritis is of utmost importance due to its significant impact on morbidity and death rates. The first action in treatment should be promptly stopping the administration of any drugs that may potentially cause UGI. Regular blood product support, including transfusions of red blood cells and administration of folate, is crucial for maintaining optimal blood counts, alleviating symptoms of gastritis and hematemesis, and enhancing overall quality of life.
Temozolomide is a cytotoxic prodrug that undergoes spontaneous conversion into its active form inside the cellular compartment, without specificity towards a particular cell phase. This mechanism involves the process of purine base methylation, which leads to the occurrence of DNA breaks and subsequent cell death (Zhang et al., 2012). Temozolomide has emerged as the established therapy modality for primary intracranial malignancies, namely anaplastic astrocytoma and glioblastoma multiforme, after enhanced progression-free survival and overall survival rates shown in several clinical studies.
TMZ has also shown favourable response rates in other malignancies such as metastatic melanoma, Ewing sarcoma, and T-cell cutaneous lymphomas, leading to its expanded use in medical oncology (Tani et al 2005). Other than temozolomide, there does not seem to be any other logical risk factor for the development of acute gastritis in our patient. There was no environmental exposure, no family history, and no potential medication-related reasons. He was just on metformin before beginning temozolomide, which has not been linked to severe gastritis. Levetiracetam was started as soon as a diagnosis was made. There have been no documented occurrences of levetiracetam-induced acute gastritis, even though some anticonvulsants have occasionally been linked to acute gastritis. Using the Naranjo method, the likelihood that the adverse drug response (ADR) was caused by TMZ was determined. A score of +5 was recorded, suggesting a likely ADR (Naranjo et al,1981).
4.Conclusion
Despite being regarded as a generally safe medication, several reports of ADRs associated with temozolomide have been documented. Establishment of a definitive causal relationship of UGI with temozolomide has been challenging due to the concurrent administration of other routinely prescribed medicines. However, there have been several recorded instances of ADR occurring in individuals who are undergoing temozolomide monotherapy.
The most effective approach for the management of acute gastritis generated by temozolomide remains uncertain, mostly owing to the rarity of instances and the variability in the manifestations and reactions seen among afflicted individuals. It is important for physicians to possess knowledge on the infrequent but life-threatening toxicity linked to temozolomide to make informed decisions when administering this medication. Regular blood tests should be conducted, both during and shortly after therapy, to facilitate early identification and management of UGI, the potentially deadly manifestation of acute gastritis.
References
- Alifieris, C., & Trafalis, D. T. (2015). Glioblastoma multiforme: Pathogenesis and treatment. Pharmacology & Therapeutics, 152, 63–82. https://doi.org/10.1016/j.pharmthera.2015.05.005
- Chamberlain, C. E. (1993). Acute hemorrhagic gastritis. Gastroenterology Clinics of North America, 22(4), 843–873.
- Gilbar, P. J., Pokharel, K., & Mangos, H. M. (2021). Temozolomide-induced aplastic anaemia: Case report and review of the literature. Journal of Oncology Pharmacy Practice : Official Publication of the International Society of Oncology Pharmacy Practitioners, 27(5), 1275–1280. https://doi.org/10.1177/1078155220967087
- Hirtz, A., Rech, F., Dubois-Pot-Schneider, H., & Dumond, H. (2020). Astrocytoma: A Hormone-Sensitive Tumor? International Journal of Molecular Sciences, 21(23). https://doi.org/10.3390/ijms21239114
- Joffe, S. N., & Rao, S. S. (1982). Symptoms of gastritis. Scandinavian Journal of Gastroenterology. Supplement, 79, 62–65.
- Lee, S. Y. (2016). Temozolomide resistance in glioblastoma multiforme. Genes & Diseases, 3(3), 198–210. https://doi.org/10.1016/j.gendis.2016.04.007
- Naranjo, C. A., Busto, U., Sellers, E. M., Sandor, P., Ruiz, I., Roberts, E. A., Janecek, E., Domecq, C., & Greenblatt, D. J. (1981). A method for estimating the probability of adverse drug reactions. Clinical Pharmacology and Therapeutics, 30(2), 239–245. https://doi.org/10.1038/clpt.1981.154
- Panarelli, N. C. (2014). Drug-induced injury in the gastrointestinal tract. Seminars in Diagnostic Pathology, 31(2), 165–175. https://doi.org/10.1053/j.semdp.2014.02.007
- Stupp, R., Mason, W. P., van den Bent, M. J., Weller, M., Fisher, B., Taphoorn, M. J. B., Belanger, K., Brandes, A. A., Marosi, C., Bogdahn, U., Curschmann, J., Janzer, R. C., Ludwin, S. K., Gorlia, T., Allgeier, A., Lacombe, D., Cairncross, J. G., Eisenhauer, E., Mirimanoff, R. O., … National Cancer Institute of Canada Clinical Trials Group. (2005). Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England Journal of Medicine, 352(10), 987–996. https://doi.org/10.1056/NEJMoa043330
- Tani, M., Fina, M., Alinari, L., Stefoni, V., Baccarani, M., & Zinzani, P. L. (2005). Phase II trial of temozolomide in patients with pretreated cutaneous T-cell lymphoma. Haematologica, 90(9), 1283–1284.
- Zhang, J., Stevens, M. F. G., & Bradshaw, T. D. (2012). Temozolomide: mechanisms of action, repair and resistance. Current Molecular Pharmacology, 5(1), 102–114. https://doi.org/10.2174/1874467211205010102
Authorship Contribution Statement:
SA, AJ, VR, SS, SSB, NS, JN, SCP, KKFD, YJ, BSN, SHP, SSMG, NS, and AM contributed significantly to the manuscript. SA led the conceptualization and writing of the manuscript. AJ, VR, SS, and SSB assisted in drafting and provided critical intellectual content. NS, JN, SCP, KKFD, and YJ contributed to manuscript revision and provided clinical insights. BSN, SHP, SSMG, NS, and AM were involved in the critical review and editing of the manuscript for important intellectual content. All authors have read and approved the final manuscript.
Ethics Approval and Consent to Participate:Proper patient consent was taken for the publication of this case report in written format according to journal guidelines.
Consent for Publication: Informed consent was obtained from the patient for publication of this case report and any accompanying images.
Availability of Data and Materials: Not applicable.
Competing Interests:The authors declare that they have no competing interests.
Funding: No funding was received for this study.
Acknowledgment:None.
Disclosure: None.


